Surface Tension
Author: Ciara Higham
What is surface tension?
Surface tension is a property of a liquid which allows it to resist an external force. For example, a paper clip can float on water when you might normally expect it to sink due to its weight. Different liquids have different surface tensions, for example, water has just over double the surface tension of sunflower oil. Can you float a paperclip on sunflower oil?

What causes surface tension?
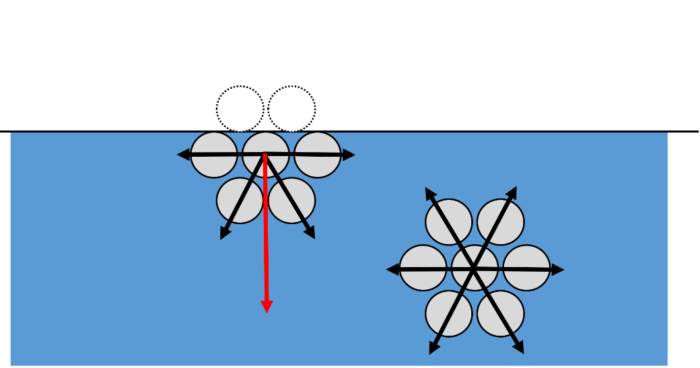
To fully understand surface tension, we must look at the molecular level. Molecules of the same type attract each other, and this attractive force is known as cohesion. At the centre of a liquid, a molecule is surrounded by lots of other molecules that are attracted to each other because of cohesion. As each molecule is surrounded by lots of other molecules, the cohesive forces are in balance. For molecules at the surface, the cohesive forces are unbalanced as there are no molecules above them. This means molecules at the surface of a liquid are pulled towards their neighbours below the surface. This creates a “skin” at the surface known as surface tension.
Bubbles, raindrops and more…
A surface that is under surface tension will take the form of the shape with smallest area. This minimises the number of molecules that are at the surface and so unbalanced forces are the least. This is why bubbles and raindrops in the air are round – a sphere has the smallest surface area when compared to any other shape of the same volume.

But raindrops on a window aren’t spherical?
Yes, droplets of liquid are not always spherical. You may have noticed this if you look at raindrops on a window. This is because there is a force between the raindrop and the window. This force is known as adhesion.

What is adhesion?
Adhesion causes molecules of different types to be attracted to each other. For a raindrop on a window, the water molecules in the raindrop are attracted to each other due to cohesion. The molecules of water near the window are attracted to the window molecules due to adhesion.
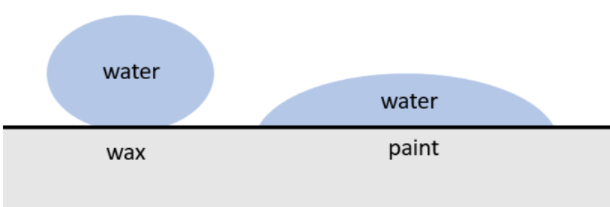
Droplets on different surfaces
Depending on the type of liquid and solid surface, the way the liquid behaves when it contacts the surface will be different. This is why water droplets form different shapes on different surfaces. On some surfaces the droplets are almost spherical, but on other surfaces the droplets spread out and become flat.
Walking on water
When we put our finger into water, we can break the surface because we are able to overcome the surface tension force easily. If we were smaller however, like an insect, this would be much harder for us to do. Some insects can take advantage of this and are able to run across the surface of ponds, like the water strider. Water striders have tiny hairs all over their legs and bodies which help to repel the water (this is almost like having waxy legs). This stops droplets of water sticking to them and weighing them down, and they can skip across the surface of the water.

